Pfizer Vaccine Data Review Summary
- Zeel Shah MD
- Dec 24, 2020
- 9 min read
Updated: Dec 24, 2020
Part 1: This post will contain information on the background of SARS-CoV2, the mechanism of action of an mRNA-based vaccine, and an overview of the NEJM article called "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine".
It seems like this is the beginning of the end of this 2020 COVID-19 Pandemic (finally, amirite?). The first vaccine was made and deployed in record time! We are truly living as history is being made.
There's an abundance of information surrounding the recently released Pfizer vaccine and I could not be more excited!
Buckle up friends and let's get started.
A little summary of our pandemic-related 2020 before we begin:
In 2019, there was a pneumonia of unknown cause that was infecting people left, right and center.
- Turns out that this pneumonia was caused by a zoonotic coronavirus which had structural similarities to SARS-CoV, a lineage B beta coronavirus.
- SARS-CoV-2 is an enveloped, positive sense, single stranded RNA virus.
- More than 70% of its sequence is shared with SARS-CoV (severe acute respiratory syndrome)
- Approximately 50% of its sequence is shared with with MERS-CoV (Middle Eastern respiratory syndrome)
What does this look like?!
Take a look at this bad boy right here in the picture below. The key thing we gotta pay attention to is the SPIKE protein (S) aka the blue spikey thing poking out of the circle.
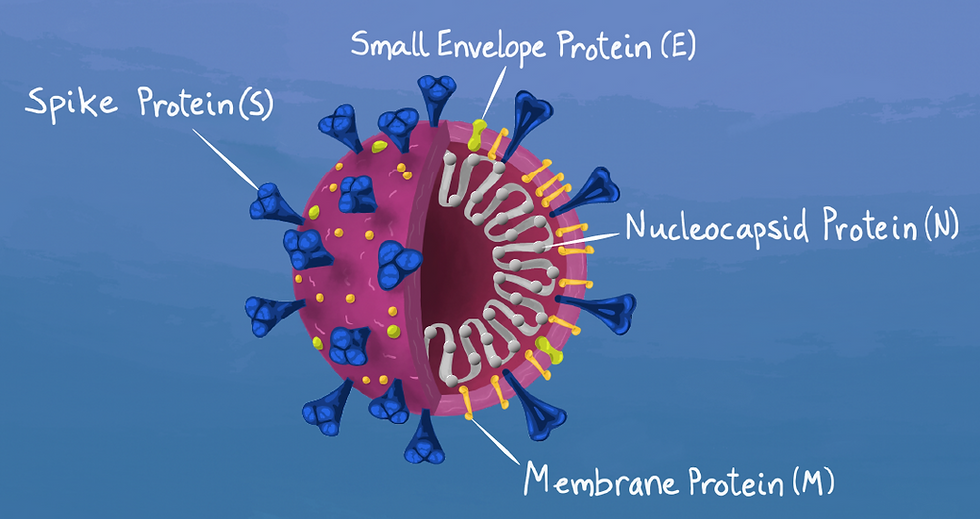
This spike protein binds to a receptor in our bodies called human angiotensin converting enzyme 2 (hACE2) and BAM we have an infection process going. You can find these bad boys (hACE-2) in the human heart, lung, kidney and gastrointestinal tract. SOOOOO this is why you have respiratory and systemic symptoms occur when infected with SARS-CoV2. I've included a simplified depiction of what the heck is happening:

Since we know that this is how the enemy attacks, when attempting to create a vaccine - we need to strategize the best mode of attack.
The Pfizer vaccine, among several other vaccine candidates, focuses on the spike protein (aka the blue pokey things) or parts of the spike protein.
What in the heck is this BNT162b2 vaccine candidate?
- Lipid nanoparticle–formulated (which is basically what the mRNA component is put in so that it can be taken up by the body...kinda like a car)
- Nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein
Okay everyone calm down. I know that was a lot of science to throw at you all at once but it might be easier to take a look at this awesome animation to really visualize it.
If you don't like science animations and prefer tweets for your information, I have a great analogy for you as well. This is an equal opportunity space and the important thing is to get an idea of how this vaccine ACTUALLY works. Whatever works, right? Right- so take your eye marbles and look below:

How did they modify the mRNA of the full-length SARS-CoV2 spike protein?
Well all you cool cats and kittens - they modified the prefusion conformation and changed it by 2 proline mutations to lock it into this formation.
How Was The Study Designed?
Now that we know what the strategy was - you're probably wondering how in the heck they designed and did this? Well friends, I'm about to tell you.
- It was structured as a placebo-controlled, observer-blinded, pivotal efficacy trial. Check out the NEJM article for further details.
- The investigators randomly assigned people 16 years of age or older in a 1:1 ratio to receive two doses, 21 days apart, of either placebo or the BNT162b2 vaccine candidate (30 μg per dose).
What were the study investigators trying to find out?
There were 2 primary endpoints and a lot of secondary endpoints. I will be focusing on the primary endpoints and if you are interested in learning more about the secondary endpoints, please check out the FDA briefing document. This data set and these trial results are the basis for an application for emergency use authorization. (We shall get more into that in another post but for now, let's focus on the NEJM article.)
Primary endpoints:
Efficacy of the vaccine against laboratory-confirmed Covid-19
Safety
What Methods Were Used?
Trial Objectives/Participants/Oversight
- Assessed safety and efficacy of two 30-ug doses of BNT162b2 administered intramuscularly 21 days apart as compared with placebo.
Who was allowed to participate?
Adults 16 years or older that were healthy or had stable chronic medical conditions (including but not limited to HIV, Hep B, Hep C) .
People that were excluded from the trial included:
People with chronic obstructive pulmonary disease (COPD)
People taking immunosuppressive therapy
People diagnosed with an immunocompromised condition.
Trial Procedures:
They used an interactive web-based system to randomly assign participants in a 1:1 ratio to receive 30uG of BNT162b2 OR saline placebo.
Participants received 2 injections, 21 days apart, of either BNT162b2 or placebo and were delivered in the deltoid muscle.
Participants were unaware of the group assignments and they observed the participants for 30 min after vaccination for any acute reactions.
Primary Endpoint: Safety
These were defined as:
Solicited, specific local or systemic adverse events (meaning they ASKED the patients about these)
Use of antipyretic or pain medication within 7 days after receiving the dose of vaccine or placebo in a subset of participants as prompted/recorded by the electronic diary —> this was known as the reactiogenicity subset
Unsolicited adverse events (which refers to events that were reported by participants without prompting from the electronic diary)
Primary Endpoint: Efficacy
First primary endpoint: efficacy of BNT162b2 against confirmed COVID19 with onset at least 7 days after the 2nd dose in participants who had been without serologic or virology evidence of SARS-CoV2 infection up to 7 days after 2nd day.
Second primary endpoint: efficacy in participants with and participants without evidence of prior infection.
How did they define someone as having COVID-19?
COVID19 definition: at least one of the following:
1. (+) fever
2. new or increased cough
3. new or increased SOB
4. chills
5. new or increased muscle pain
6. new loss of taste or smell
7. sore throat, diarrhea or vomiting
AND a respiratory specimen that was obtained during the symptomatic period OR within 4 days before or after it was COVID(+) by nucleic acid amplification based testing.
These were other key characteristics that we should be mindful of:
- Safety population includes people ≥ 16 years old
- The total number of participants that were enrolled and injected with either the vaccine or the placebo were 43,448
- The number of people that would end up being evaluated for efficacy 7 days after the second dose and had no evidence of prior infection was 36,523
- The number of people that could be evaluated 7 days after the second dose with or without evidence of prior infection was 40,137
Statistical Analysis
Safety analysis included all participants that received at least 1 dose of BNT162b2 or placebo.
Findings are not based on formal statistical hypothesis testing but rather are descriptive in nature.
Analysis of first primary efficacy end point included participants who received the vaccine or placebo as randomly assigned: no evidence within 7 days after 2nd dose and had no major protocol deviation.
Vaccine efficacy was estimated by 100x(1-IRR) where IRR is the calculated ratio of confirmed cases of COVID19 per 1000 person-years of follow up in the active vaccine group to the corresponding illness rate in the placebo group.
The 95% credible interval for vaccine efficacy and the probability of vaccine efficacy ≥ 30% were calculated with the use of a Bayesian beta-binomial model.
Final analysis uses a success boundary of 98.6% for probability of vaccine efficacy ≥ 30% to compensate for the interim analysis and to control the overall type 1 error rate at 2.5%.
Primary and secondary efficacy end points are evaluated sequentially to control the familywise type 1 error rate at 2.5%.
I know that was a whole lot of numbers there but its an important summary of key points discussed in the methods.
Now, what EVERYONE'S BEEN WAITING FOR!!! *drumroll please*
Results
I will be primarily focusing on the figures discussed in the New England Journal of Medicine along with a summary of the key points. This is not meant to be a full thorough analysis so please click on the link and check out the actual article.
Figure 1. Enrollment and Randomization.

Check out this graphic of all the enrolled participants through Nov. 14, 2020.
The safety subset is based on a data cut-off date was Oct. 9, 2020. They did this to follow the application requirements for the Emergency Use Authorization.
In case anyone was wondering- the procedures that 1 participant in the placebo group declined after Dose 2 was the collection of blood and nasal swap samples. (lower right corner)
Some of you might be wondering - hmmm, so what was the composition of these participants? Well, check out this awesome table:

Of the 37,706 participants:
49% were female
83% were White
9% were Black or African American
28% were Hispanic or Latinx
35% were obese (body mass index [the weight in kilograms divided by the square of the height in meters] of at least 30.0)
21% had at least one coexisting condition
The median age was 52 years
42% of participants were older than 55 years of age
Now the elephant in the room - IS THIS SAFE?! Follow me as we go through the local and systemic reactogenicity, adverse effects, and efficacy. Get ready for some more fun figures and tables.
Local and Systemic Reactogenicity
I know this looks like a busy figure but don't worry - we'll break it down.

Let's start with Figure 2A - this one shows us the LOCAL events that took place.

There were 8,183 participants that were part of the reactogenicity subset.
Generally, the people that received the vaccine had more local reactions than the placebo recipient.
The most commonly reported local reaction was mild-to-moderate pain at the injection site within 7 days. Less than 1% of the participants across all age groups reported severe pain.
In recipients older than 55years of age: pain was reported less frequently.
- 71% after the first dose
- 66% after the second dose
This was less than the younger recipients:
- 83% after the first dose
- 78% after the second dose.
A lower percentage of people reported injection-site redness or swelling.
Interestingly, the proportion of people reporting local reactions did NOT increase after the second dose.
No participants reported a grade 4 (which means REALLY BAD) local reaction.
Most of them were mild-to-moderate in severity and resolved within 1-2 days.
Let's move onto the SYSTEMIC side effects:

Most of the systemic symptoms were seen in younger vaccine recipients (16 to 55 years of age) compared to older vaccine recipients (≥55 years of age). These also tended to be seen more often after dose 2 than dose 1.
Most commonly reported systemic events:
Fatigue and headache: (59% and 52%, respectively, after the second dose, among younger vaccine recipients; 51% and 39% among older recipients)
Although fatigue and headache were also reported by many placebo recipients (23% and 24%, respectively, after the second dose, among younger vaccine recipients; 17% and 14% among older recipients).
The frequency of any severe systemic event after the first dose was 0.9% or less.
Severe systemic events were reported in less than 2% of vaccine recipients after either dose, except for fatigue (in 3.8%) and headache (in 2.0%) after the second dose.
Fever (temperature, ≥38°C) was reported after the second dose by 16% of younger vaccine recipients and by 11% of older recipients.
- Only 0.2% of vaccine recipients and 0.1% of placebo recipients reported fever
(temperature, 38.9 to 40°C) after the first dose, as compared with 0.8% and 0.1%,
respectively, after the second dose.
- Two participants each in the vaccine and placebo groups reported temperatures
above 40.0°C.
** Younger vaccine recipients were more likely to use antipyretic or pain medication (28% after dose 1; 45% after dose 2) than older vaccine recipients (20% after dose 1; 38% after dose 2), and placebo recipients were less likely (10 to 14%) than vaccine recipients to use the medications, regardless of age or dose.
Systemic symptoms including fever and chills were observed within the first 1 to 2 days after vaccination and resolved shortly thereafter.
Adverse Event Analysis
More BNT162b2 recipients than placebo recipients reported any adverse event (27% and 12%, respectively) or a related adverse event (21% and 5%).
This is likely reflective of the transient reactiogenicity events and vaccine recipients more commonly reported these as adverse events compared to the placebo group.
Lymphadenopathy: Sixty-four vaccine recipients (0.3%) and 6 placebo recipients (<0.1%)
Only 4 related serious adverse events were reported among BNT162b2 recipients: shoulder injury related to vaccine administration, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia.
2 of the vaccine recipients died (one from arteriosclerosis, one from cardiac arrest), as did 4 placebo recipients (two from unknown causes, one from hemorrhagic stroke, and one from myocardial infarction).
No deaths were considered by the investigators to be related to the vaccine or placebo. No Covid-19–associated deaths were observed.
No stopping rules were met during the reporting period.
Safety monitoring will continue for 2 years after administration of the second dose of vaccine.
Efficacy
Figure 3. Efficacy of BNT162b2 against Covid-19 after the First Dose.

Among 36,523 participants who had no evidence of existing or prior SARS-CoV-2 infection, 8 cases of Covid-19 with onset at least 7 days after the second dose were observed among vaccine recipients and 162 among placebo recipients.
Shown is the cumulative incidence of Covid-19 after the first dose (modified intention-to-treat population).
Each symbol represents Covid-19 cases starting on a given day; filled symbols represent severe Covid-19 cases.
Some symbols represent more than one case, owing to overlapping dates.
The time period for Covid-19 case accrual is from the first dose to the end of the surveillance period.
The confidence interval (CI) for vaccine efficacy (VE) is derived according to the Clopper–Pearson method. If you're not sure what that means - you're in luck because neither do I. If any statisticians can explain this method - love to hear it. I think the main point is that there was a regimented equation used to determine the confidence interval and it didn't just pop out of thin air.
In the next blog post, I will be going through an interesting discussion of this article, the editors note, and delve further into the diagrams mentioned in the FDA briefing document.
I hope you enjoyed this blog posts and found it to be educational and engaging!
Until next time,
- Z
Noteworthy References
There are some excellent summaries out there that I enjoyed listening to and reading. I think the best summary on YouTube that I've seen is by Dr. F Perry Wilson and he does a fantastic job of going through the FDA document in exceptional detail. I highly recommend watching this in its entirety and I will be referencing his analysis from this video in my blog post.
Another great resource is Deplatform Disease by Edward Nirenberg and would highly recommend subscribing to his blog.
Of course, I used the New England Journal of Medicine quite heavily (obviously) and thank them for publishing this paper.





Comments